Weld-Metal Protection
 22 мая, 2014
22 мая, 2014  Oleg Maloletnikov
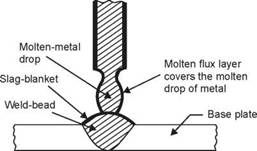
Oleg Maloletnikov • Flux melts with the core wire and covers the surface of the molten metal drops and the weld-pool (see Fig. 4.3), excluding oxygen and nitrogen to come in their contact. As the weld-pool progressively solidifies, the flux forms a slag blanket over the weld - bead and continues to protect it from oxidation till it cools to room temperature.
|
Fig. 4.3 Molten flux covers molten metal droplet and forms a slag blanket over the weld bead excluding oxygen and nitrogen to come in their contact |
• The flux must also be completely detachable. This is very important especially when multiple layers are to be deposited. Ideally we require a slag which automatically detaches itself off the weld deposit. This requirement is difficult to reconcile with the need to adhere to the weld-metal during the cooling period. Slag detachability is also influenced by compounds added to the flux to achieve other objectives. A compromise between the antagonistic effects of the compounds added to achieve different objectives is the only solution.
• Additional protection from atmospheric oxygen and nitrogen contamination is provided by adding compounds in the coating which decompose by the heat of the arc and form an additional gaseous shield around the arc and weld-pool. They may be carbonates (giving carbon dioxide) or cellulose (giving hydrogen and carbon monoxide).
4.3.1 Arc Stability
• There are two major aspects of arc stability. It is the ease of initiating and maintaining an electric arc during welding, and reigniting the arc during each half cycle in a. c. welding. For this to occur the gases in the arc gap must ionise rapidly and at lowest possible potential. Additions of titanium oxide, potassium silicate, calcium carbonate facilitate arc stabilisation. This is in addition to their normal purpose of acting as a flux.
Thus arc stability depends upon:
— O. C.V. of power source
— Transient voltage recovery characteristics of the power source
— Size of molten drops of filler metal and slag in the arc
— Arc path ionisation
— Electrode manipulation
A stable arc is also the one which is maintained straight along the electrode, axis and does not waver to find the shortest path especially on the sides of a vee edge preparation during welding in a groove, i. e. it must stay firmly fixed in the direction dictated by the welder.
4.3.2 Control of Weld-Metal Composition
This is one of the advantages of SMAW that it permits the control of weld metal composition by adding alloying elements to the flux covering. From a given combination of flux and weld - metal compositions, the alloying elements are distributed between the two in more-or-less the same proportion. If the flux or slag is low in, say, manganese, this metal transfers from the weld to the slag until the correct proportion is reached. Thus elements can be added to or taken from the weld deposit simply by altering the flux composition. The amounts of alloying elements to be added to produce a particular weld-metal composition can be calculated by the electrode manufacturer. In general, there are three major factors that control weld-metal composition. These are: alloying, deoxidation, and contamination control.
Alloying. When the core wire used has the same composition as desired in the weld, we need not add any alloying elements, except to ensure that the elements are not lost during welding. The electrodes used with low carbon, carbon-manganese, and low alloy steels, alloyed core wires turn out to be expensive. Alloying is to be done in the weld pool. Thus low carbon steel core wires could be used and manganese, chromium, molybdenum, etc. could be added through the flux. This helps in producing a large variety of electrodes with the same core wire, especially when small quantities of specific composition are needed.
Deoxidation. During the welding of steel, if the molten weld-metal pool contains excessive oxygen, it gives rise to the formation of carbon monoxide bubbles which get trapped in the solidifying weld metal to form porosity:
FeO + C = Fe + CO
This also causes loss of carbon which reduces the strength of the weld. This reaction can be supressed by adding deoxidants in the coating. A commonly used deoxidant for steel is silicon (added to the coating as ferro-silicon). Oxygen reacts with silicon in preference to steel as follows:
2FeO + Si = 2Fe + SiO2
Silicon oxide formed floats to the weld-pool surface and forms slag. For welding copper the deoxidant used could be phosphorus or zinc to remove the oxygen and could be added to the filler metal and not to flux.
Contamination. The most harmful contaminant entering the molten weld-pool through the flux is hydrogen which leads to the formation of hydrogen cracks. Hydrogen is present in the electrode flux covering both as combined and absorbed moisture. Absorbed moisture can be removed by drying the electrodes before welding. The extent of chemically combined moisture depend upon the compounds used in the coating. Hydrogen has very high solubility in iron at elevated temperature. As the metal solidifies the solubility goes down and hydrogen bubbles are formed and are entrapped. As the metal cools and contracts, the pressure in the bubble exceeds the metal strength at that temperature forming cracks. Oxidising iron-oxide electrodes have been found to give beneficial results in solving the problem of hydrogen cracking.
• Other contaminants could be due to careless handling of the electrodes. Grease, oil, damped sulphurous fumes absorbed from the surroundings etc. may be transferred to the weld pool and cause harm. Careful handling of electrodes is, therefore, necessary.

 Опубликовано в
Опубликовано в